Publications
119. Total Synthesis of Feglymycin Using Umpolung Amide Synthesis Preston C. Gourville, Jade A. Bing, Rashanique D. Quarels, Sergey V. Tsukanov, Kenneth E. Schwieter, Kazuyuki Tokumaru, Amanda B. Stephens, Dawn M. Makley, Bo Shen, Abigail N. Smith, Jeffrey N. Johnston* Angew. Chem. Int. Ed. Engl. 2025, 64, e202508819.
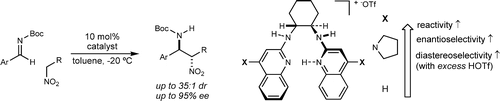
118. Performance Enhancing Asymmetric Catalysis Driven by Achiral Counterion Design Zihang Deng, Jenna L. Payne, Mahesh Vishe, Julius E. L. Jan, Cody M. Funk, Jeffrey N. Johnston* J. Am. Chem. Soc. 2025, 147, 17584-17591
Highlighted by Dirk Trauner & Michael D. Zott in Synfacts
117. End-to-End Backbone Cyclization Enhances Passive Permeability of bRo5 Oligomeric Depsipeptides with Nonlinear Size Dependence Madelaine Thorpe, Corey R. Hopkins, Jeffrey N. Johnston* ACS Med. Chem. Lett. 2025 16, 638-645
116. Elucidating Fluorine Steering Effects in Diels-Alder Reactions Interfaced with Charge-Enhanced Reactivity Sabrina Hoford, Julius E. L. Jan, Jeffrey N. Johnston,* Travis Dudding* Eur. J. Org. Chem. 2025, e202401203
115. The Backbone Constitution Drives Passive Permeability Independent of Side Chains in Depsipeptide and Peptide Macrocycles
Madelaine P. Thorpe, Abigail N. Smith, Daniel J. Blackwell, Corey R. Hopkins, Bjorn C. Knollmann, Wendell S. Akers, and Jeffrey N. Johnston*
Chem. Sci. 2024, 15, 14977-14987.
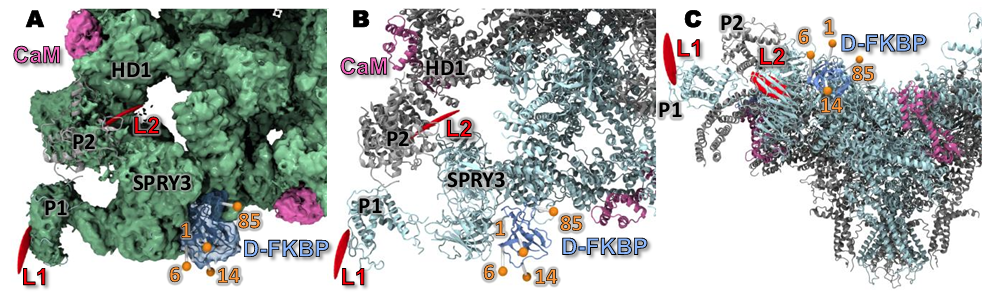
114. Backbone-Determined Antiarrhythmic Structure-Activity Relationships for a Mirror-Image, Oligomeric Depsipeptide Natural Product
Madelaine P. Thorpe, Daniel J. Blackwell, Bjorn C. Knollmann, and Jeffrey N. Johnston*
J. Med. Chem. 2024, 67, 12205-12220
Special issue for Natural Products Driven Medicinal Chemistry
113. Generality-Driven Catalyst Development: A Universal Catalyst for Enantioselective Nitroalkene Reduction
Zihang Deng, Melanie A. Padalino, Julius E. L. Jan, Sangjun Park, Michael W. Danneman, and Jeffrey N. Johnston*
J. Am. Chem. Soc. 2024, 146, 1269-1275.
Highlighted by Winter et al. in Trendbericht: Organische Chemie 2025
Highlighted by Douglass Taber at Organic Chemistry Highlights
Highlighted by Reto Mueller at the Organic Chemistry Portal
112. ent-Verticilide B1 inhibits type 2 ryanodine receptor channels and is antiarrhythmic in Casq2-/- mice
Gochman, A.; Do, T. Q.; Kim, K.; Schwarz, J. A.; Thorpe, M. P.; Blackwell, D. J.; Ritschel, P.; Smith, A. N.; Rebbeck, R. T.; Akers, W. S.; Cornea, R. L.; Laver, D. R.; Johnston, J. N.; Knollmann, B. C.*
bioRxiv 2023 & Mol. Pharm. 2024, 105, 194-201.
111. RyR2 Binding of an Antiarrhythmic Cyclic Depsipeptide Mapped Using Confocal Fluorescence Lifetime Detection of FRET
Šeflová, J.; Schwarz, J. A.; Smith, A. N.; Svensson, B.; Blackwell, D. J.; Phillips, T. A.; Nikolaienko, R.; Bovo, E.; Rebbeck, R. T.; Zima, A. V.; Thomas, D. D.; Van Petegem, F.; Knollmann, B. C.; Johnston, J. N.*; Robia, S. L.; Cornea, R. L.*
ACS Chem. Biol. 2023, 18, 2290-2299.
110. The selective RyR2 inhibitor ent-verticilide suppresses atrial fibrillation susceptibility caused by Pitx2 deficiency
Kim, K. ; Blackwell, D.J. ; Yuen, S.L. ; Thorpe, M. P. ; Johnston, J. N. ; Cornea, R.L. ; Knollmann, B. C.*
J. Mol. Cell. Cardiol. 2023, 180, 1-9.
“Top Original Research Paper of the Year” by the Editors of the J. Mol. Cell. Cardiol. for 2023
Highlighted by Leigh MacMillan in a VUMC Reporter spotlight.
109. In vivo pharmacokinetic and pharmacodynamic properties of the antiarrhythmic molecule ent-verticilide
Blackwel, D.; Smith, A.; , Do, T. Q.; Gochman, A.; Schmeckpeper, J.; Hopkins, C.; Akers, W.*; Johnston, J. N.*; Knollmann, B.*
J. Pharm. Exptl. Ther. 2023, 385, 205-213.
108. Enantioselective Synthesis of cis- and trans-Cycloheptyl β‑Fluoro 2 Amines by Sequential aza-Henry Addition/Ring-Closing Metathesis
Bing, J. A. ; Johnston, J. N.
Org. Lett. 2023, 25, 950-955.
107. A Secondary Orbital Effect Involving Fluorine is Responsible for Substrate-Controlled Diastereodivergence in the syn-aza-Henry Reaction of α-Fluoronitroalkanes
Smajlagic, I. ; Johnston, J. N. ; Dudding, T.
Chem. Eur. J. 2023, 29 (24), e202204066.
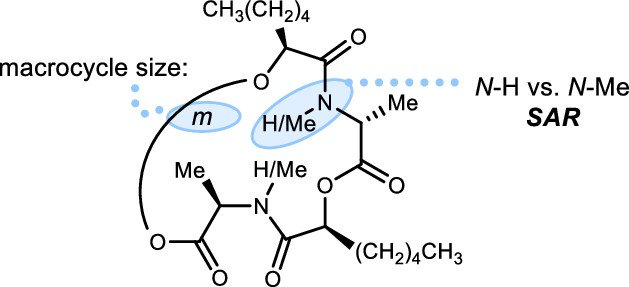
106. Structure-Activity Relationships for the N-Me- versus N-H-Amide Modification to Macrocyclic ent-Verticilide Antiarrhythmics
Smith, A.; Thorpe, M; Blackwell, D; Batiste, S; Hopkins, C; Schley, N; Knollmann, B; Johnston, J. N.
ACS Med. Chem. Lett. 2022, 13, 1755-1762.
Featured in the Virtual Collection - Future of Medicinal Chemistry Collection
Don’t miss the Editorial, including statements from Abby (and Jeff) - Special Collection Editorial
105. Preparation of N-Aryl Amides by Epimerization-Free Umpolung Amide Synthesis
Crocker, M. S.; Deng, Z. Johnston, J. N.
J. Am. Chem. Soc. 2022, 144, 16708-16714.
Featured by Douglass Taber in Org. Chem. Highlights - spectacular reactions
104. Enantioselective Iodolactonization to Prepare ε-Lactone Rings Using Hypervalent Iodine
Payne, J.; Deng, Z.; Flach, A.; Johnston, J. N.* [†equal contribution]
Chem. Sci. 2022, 13, 7318-7324.
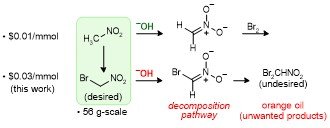
103. Resolving Bromonitromethane Sourcing by Synthesis: Preparation at the Decagram Scale
Thorpe, M. P.; Smith, A. N.; Crocker, M. S.;Johnston, J. N.
J. Org. Chem. 2022, 87, 5451-5455.
102. Exercise Causes Arrhythmogenic Remodeling of Intracellular Calcium Dynamics in Plakophilin-2 Deficient Hearts
van Opbergen, C.; Bagwan, N.; Maurya, S; Kim, J.C.; Smith, A; Blackwell, D; Johnston, J.N.; Knollmann, B; Cerrone, M.; Lundby, A; Delmar, M.
Circulation 2022, 145, 1480-1496.
Highlighted in Muscle Cell News
101. Fluorine-Induced Diastereodivergence Discovered in an Equally Rare Enantioselective syn-aza-Henry Reaction
Bing, J. A.; Schley, N. D.; Johnston, J. N.
Chem. Sci. 2022, 13, 2614-2623.
100. Ring-Size as an Independent Variable in Cyclooligomeric Depsipeptide Antiarrhythmic Activity
Smith, A. N.; Blackwell, D. J.; Knollmann, B. C.; Johnston, J. N.
ACS Med. Chem. Lett. 2021, 12, 1942-1947.
99. DFT-Based Stereochemical Rationales for the Bifunctional Brønsted Acid/Base-Catalyzed Diastereodivergent and Enantioselective aza-Henry Reactions of α-Nitro Esters
Struble, T. J.;† Foy, Hayden;† Dudding, T.*; Johnston, J.N. [†equal contribution]
J. Org. Chem. 2021, 86, 15606-15617.
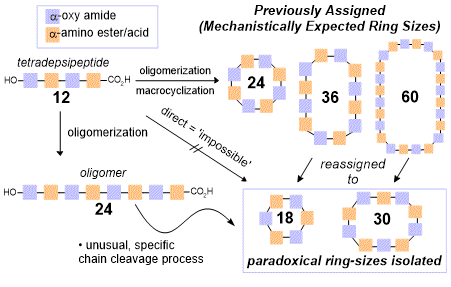
98. The Formation of Impossible Rings in Macrocyclooligomerizations for Cyclodepsipeptide Synthesis: The 18-From-12 Paradox
Smith, A. N.; Johnston, J. N..
J. Org. Chem. 2021, 86, 7904-7919.
Related: Batiste, S. M.; Johnston, J. N., Correction to “Evidence for Ion-Templation During Macrocyclooligomerization of Depsipeptides”. J. Am. Chem. Soc. 2021, 143, 6701.
Related: Correction to “Rapid synthesis of cyclic oligomeric depsipeptides with positional, stereochemical, and macrocycle size distribution control” here.
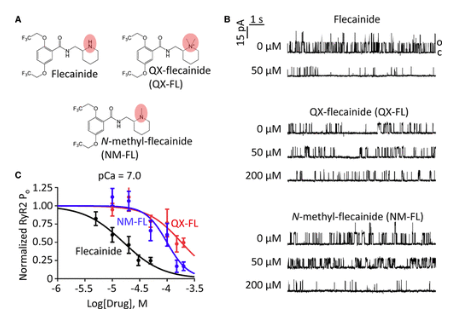
97. RYR2 Channel Inhibition is the Principal Mechanism of Flecainide Action in CPVT
Kryshtal, D. O.; Blackwell, D. J.; Egly, C. L.; Smith, A. N.; Batiste, S. M.; Johnston, J. N.; Laver, D. R.; Knollmann, B. C
Circ. Res. 2021, 128, 321.
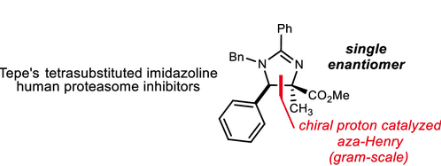
96. Substituted Imidazoline Synthesis: A Diastereo- and Enantioselective aza-Henry Route to a Human Proteasome Modulator
Sprague, D. J.; Johnston, J. N.
Org. Lett. 2020, 22, 8496-8499.
Highlighted by P. Kocienski: Synfacts 2021, 17, 17
95. Bromonitromethane: Second Update
Vishe, M. N. ; Johnston, J. N.
Encyclopedia of Reagents for Organic Synthesis 2019.
94. Direct Observation and Analysis of the Halo-Amino-Nitro Alkane Functional Group
Crocker, M. ; Foy, H. ; Tokumaru, K. ; Dudding, T. ; Pink, M. ; Johnston, J. N.
Cell: Chem 2019, 5, 1248-1264
Highlighted by Carol Rouzer in VICB News
93. Unnatural Verticilide Enantiomer Inhibits Type 2 Ryanodine Receptor Mediated Calcium Leak and is Antiarrhythmic
Batiste, S. ; Blackwell, D. ; Kim, K. ; Gomez-Hurtado, N. ; Rebbeck, R. ; Cornea, R. ; Johnston, J. N. ; Knollmann, B.
Proc. Natl. Acad. Sci. 2019, 116, 4810-4815.
Highlighted by Tien Nguyen in Chemical & Engineering News
Highlighted by Carol Rouzer in VICB News
Highlighted by Heidi Hall in Research News Vanderbilt
92. Catalytic, Enantioselective Synthesis of Cyclic Carbamates from Dialkyl Amines by CO2-Capture: Discovery, Development, and Mechanism
Yousefi, R. ; Struble, T. ; Payne, J. L. ; Vishe, M. N. ; Schley, N. D. ; Johnston, J. N.
J. Am. Chem. Soc. 2019, 141, 618-625.
Highlighted by Carol Rouzer in VICB News
91. Canvass: A Crowd-Sourced, Natural-Product Screening Library for Exploring Biological Space
Kearney, S. E. ; Many ; Other ; Authors ; Johnston, J. N. ; Rohde, J. M.
ACS Cent. Sci. 2019, 4, 1727-1741.
90. Enantioselective Organocatalytic Amine-Isocyanate Capture-Cyclization: Regioselective Alkene Iodoamination for the Synthesis of Chiral Cyclic Ureas
Struble, T. ; Lankswert, H. ; Pink, M. ; Johnston, J. N.
ACS Catal. 2018, 8, 11926-11931.
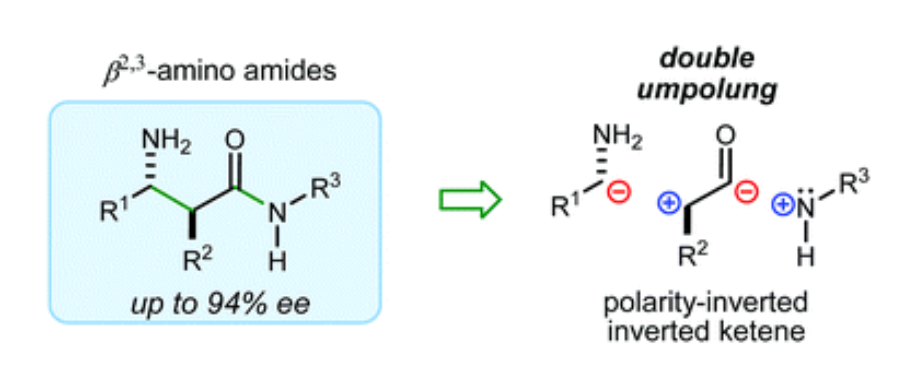
89. The Inverted Ketene Synthon: A Double Umpolung Approach to Enantioselective β2,3-Amino Amide Synthesis
Vishe, M. N. ; Johnston, J. N.
Chem. Sci. 2019, 10, 1138-1143.
Highlighted by Ben List & Joyce Grimm in Synfacts
Highlighted by Catherine Hodges in ChemistryWorld
88. Continuous Platform To Generate Nitroalkanes On-Demand (in Situ) Using Peracetic Acid-Mediated Oxidation in a PFA Pipes-in-Series Reactor
Tsukanov, S. V. ; Johnson, M. D. ; May, S. A. ; Kolis, S. P. ; Yates, M. H. ; Johnston, J. N.
Org. Process Res. Dev. 2018, 22, 971-977.
87. Evidence for Ion-Templation During Macrocyclooligomerization of Depsipeptides
Batiste, S. ; Johnston, J. N.
J. Am. Chem. Soc. 2018, 140, 4560-4568.
Highlighted by Carol Rouzer in VICB News
86. Cluster Preface: Alkene Halofunctionalization
Johnston, J. N. ; Rovis, T.
SynLett 2018, 29, 399-400.
85. Biomimetic Desymmetrization of a Carboxylic Acid
Knowe, M. ; Danneman, M. ; Sun, S. ; Pink, M. ; Johnston, J. N.
J. Am. Chem. Soc. 2018, 140, 1998-2001.
Highlighted by Organic Chemistry Highlights: https://www.organic-chemistry.org/Highlights/2018/17December.shtm
84. Diastereo- and Enantioselective Additions of α-Nitro Esters to Imines for anti-α,β-Diamino Acid Synthesis with α-Alkyl-Substitution
Sprague, D. ; Singh, A. ; Johnston, J. N.
Chem. Sci. 2018, 9, 2336-2339.
83. Preparation of Benzyl((R)-2-(4-(benzyloxy)phenyl)-2-((tert-butoxycarbonyl)amino)acetyl)-D-phenylalaninate using Umpolung Amide Synthesis
Knowe, M. ; Tsukanov, S. V. ; Johnston, J. N.
Org. Synth. 2017, 94, 388-398.
82. MDM2 Antagonists Counteract Drug-Induced DNA Damage
Vilgelm, A. ; Vara, B. ; Johnston, J. N. ; Richmond, A. ; et al.
EBioMedicine 2017, 24, 43-55.
81. 1,3,4-Oxadiazole and Heteroaromatic-Fused 1,2,4-Triazole Synthesis using Diverted Umpolung Amide Synthesis
Tokumaru, K. ; Bera, K. ; Johnston, J. N.
Synthesis 2017, 49, 4670-4675.
Thematic Issue on Heterocycle Synthesis, edited by E. Carreira
80. A Convergent Synthesis of 1,3,4-Oxadiazoles from Acyl Hydrazides Under Semiaqueous Conditions
Tokumaru, K. ; Johnston, J. N.
Chem. Sci. 2017, 8, 3187-3191.
79. Rapid Synthesis of Cyclic Oligomeric Depsipeptides with Positional, Stereochemical, and Macrocycle Size-Distribution Control
Batiste, S. ; Johnston, J. N.
Proc. Natl. Acad. Sci. 2016, 113, 14893-14897.
Highlighted by Carol Rouzer in VICB News
Highlighted by David Salisbury in Research News@Vanderbilt
Highlighted by Phys.org
78. On-Demand Complex Peptide Synthesis: An Aspirational (and Elusive?) Goal for Peptide Synthesis
Schwieter, K. E. ; Johnston, J. N.
J. Am. Chem. Soc. 2016, 138, 14160-14169.
77. Enantioselective Synthesis of β-Fluoro Amines via β-Amino α-Fluoro Nitroalkanes and a Traceless Activating Group Strategy
Vara, B. ; Johnston, J. N.
J. Am. Chem. Soc. 2016, 138, 13794-13797.
76. Enantioselective Synthesis of α-Bromonitroalkanes for Umpolung Amide Synthesis: Preparation of tert-Butyl ((1R)-1-(4-(benzyloxy)phenyl)-2-bromo-2-nitroethyl)carbamate
Lim, V. ; Tsukanov, S. V. ; Doody, A. ; Johnston, J. N.
Org. Synth. 2016, 93, 88-99.
75. Development of an Intermittent-Flow Enantioselective Aza-Henry Reaction Using an Arylnitromethane and Homogeneous Bronsted Acid−Base Catalyst with Recycle
Tsukanov, S. V. ; Johnston, M. D. ; May, S. A. ; Rosemeyer, M. ; Watkins, M. A. ; Kolis, S. P. ; Yates, M. H. ; Johnston, J. N.
Org. Process Res. Dev. 2016, 20, 215-226.
74. A One-Pot Amidation of Primary Nitroalkanes
Schwieter, K. E. ; Johnston, J. N.
Chem. Commun. 2016, 52, 152-155.
73. Enantioselective Addition of Bromonitromethane to Aliphatic N-Boc Aldimines Using a Homogeneous Bifunctional Chiral Organocatalyst
Schwieter, K. E. ; Johnston, J. N.
ACS Catal. 2015, 5, 6559-6562.
72. A Unified Approach to the Four Azaindoline Families by Inter-/Intramolecular Annulative Diamination of Vinylpyridines
Danneman, M. ; Hong, K. ; Johnston, J. N.
Org. Lett. 2015, 17, 3806-3809.
71. Enantioselective Small Molecule Synthesis by Carbon Dioxide Fixation using a Dual Brønsted Acid Base Organocatalyst
Vara, B. ; Struble, T. ; Wang, W. ; Dobish, M. ; Johnston, J. N.
J. Am. Chem. Soc. 2015, 137, 7302-7305.
70. Oxidative Inter-/Intermolecular Alkene Diamination of Hydroxy Styrenes with Electron Rich Amines
Danneman, M. ; Hong, K. ; Johnston, J. N.
Org. Lett. 2015, 17, 2558-2561.
69. Enantioselective Synthesis of D-α-Amino Amides from Aliphatic Aldehydes
Schwieter, K. E. ; Johnston, J. N.
Chem. Sci. 2015, 6, 2590-2595.
Highlighted by Matteo Zanda in Synform 2015, 8, A111
Highlighted by Douglass Taber in Organic Chemistry Portal
68. Adaptation of a Small Molecule Hydrogen Bond-Donor Catalyst to an Enantioselective Hetero-Diels-Alder Reaction Hypothesized for Brevianamide Biosynthesis
Sprague, D. ; Nugent, B. M. ; Yoder, R. A. ; Vara, B. ; Johnston, J. N.
Org. Lett. 2015, 17, 880-883.
ACS Editor's Choice - Open Access
67. Mdm3 and Aurora Kinase A Inhibitors Synergize to Block Melanoma Growth by Driving Apoptosis and Immune Clearance of Tumor Cells
Vilgelm, A. E. ; Liu, Y. ; Hawkins, O. E. ; Davis, T. ; Smith, J. ; Weller, K. P. ; Horton, L. W. ; McClain, C. M. ; Ayers, G. D. ; Turner, D. C. ; Essaka, D. C. ; Stewart, C. F. ; Sosman, J. A. ; Kelley, M. C. ; Ecsedy, J. A. ; Johnston, J. N. ; Richmond, A.
Cancer Res 2015, 75, 181-193.
66. Brønsted Acid-Catalyzed Phosphoramidic Acid Additions to Alkenes: Diastereo- and Enantioselective Halogenative Cyclizations for the Synthesis of C- and P-Chiral Phosphoramidates
Toda, Y. ; Pink, M. ; Johnston, J. N.
J. Am. Chem. Soc. 2014, 136, 14734-14737.
65. Umpolung Amide Synthesis Using Substoichiometric NIS and Oxygen as a Terminal Oxidant
Schwieter, K. E. ; Shen, B. ; Shackleford, J. ; Leighty, M. ; Johnston, J. N.
Org. Lett. 2014, 16, 4714-4717.
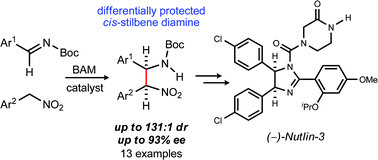
64. Organocatalytic, Diastereo- and Enantioselective Synthesis of Nonsymmetric cis-Stilbene Diamines: A Platform for the Preparation of Single Enantiomer cis-Imidazolines for Protein-Protein Inhibition
Vara, B. ; Mayasundari, A. ; Tellis, J. ; Danneman, M. ; Arredondo, V. ; Davis, T. ; Min, J. ; Finch, K. ; Guy, R. K. ; Johnston, J. N.
J. Org. Chem. 2014, 79, 6913-6938.
63. Alkene Diamination Using Electron Rich Amines: Hypervalent Iodine-Promoted Inter/Intramolecular C-N Bond Formation
Hong, K. ; Johnston, J. N.
Org. Lett. 2014, 16, 3804-3807.
62. Silyl Imine Electrophiles in Enantioselective Catalysis: A Rosetta Stone for Peptide Homologation, Enabling Diverse N-Protected Aryl Glycines from Aldehydes in Three Steps
Makley, D. ; Johnston, J. N.
Org. Lett. 2014, 16, 3146-3149.
61. Preparation of (−)-Nutlin-3 Using Enantioselective Organocatalysis at Decagram Scale
Davis, T. ; Vilgelm, A. E. ; Richmond, A. ; Johnston, J. N.
J. Org. Chem. 2013, 78, 10605-10616.
Highlighted by 'Synthesis of (-)-Nutlin-3' Kocienski, P. Synfacts 2014, 10, 1 [doi: 10.1055/s-0033-1340328]
60. Serratezomine A
Pigza, J. A. ; Johnston, J. N.
2012, 0, 131.
59. Total Synthesis of the Lycopodium Alkaloid Serratezomine A Using Free Radical-Mediated Vinyl Amination to Prepare a β-Stannyl Enamine Linchpin
Pigza, J. A. ; Han, J. ; Chandra, A. ; Mutnick, D. ; Pink, M. ; Johnston, J. N.
J. Org. Chem. 2013, 78, 822-843.
Selected as JOC Featured Article by Editors
58. VNI cures the acute and chronic experimental Chagas disease
Villalta, F. ; Dobish, M. ; Nde, P. N. ; Kleshchenko, Y. Y. ; Hargrove, T. Y. ; Johnson, C. A. ; Waterman, M. R. ; Johnston, J. N. ; Lepesheva, G. I.
J. Infect. Dis. 2013, 208, 504-511.
57. Organocatalytic, Enantioselective Synthesis of VNI: A Robust Therapeutic Development Platform for Chagas, a Neglected Tropical Disease
Dobish, M. ; Villalta, F. ; Waterman, M. R. ; Lepesheva, G. I. ; Johnston, J. N.
Org. Lett. 2012, 14, 6322-6325.
Highlighted by 'Synthesis of (+)-VNI' Kocienski, P. Synfacts 2013, 9, 250 [doi: 10.1055/s-0032-1318124]
56. Enantioselective Synthesis of α-Oxy Amides via Umpolung Amide Synthesis
Leighty, M. ; Shen, B. ; Johnston, J. N.
J. Am. Chem. Soc. 2012, 134, 15233-15236.
Highlighted by 'Synthesis of LY411575' Kocienski, P. Synfacts 2012, 8, 1293 [doi: 10.1055/s-0032-1317560]
55. Chiral Proton Catalysis of Secondary Nitroalkane Additions to Azomethine: Synthesis of a Potent GlyT1 Inhibitor
Davis, T. ; Danneman, M. ; Johnston, J. N.
Chem. Commun. 2012, 48, 5578-5580.
54. Achiral Counterion Control of Enantioselectivity in a Brønsted Acid Catalyzed Iodolactonization
Dobish, M. ; Johnston, J. N.
J. Am. Chem. Soc. 2012, 134, 6068-6071.
Highlighted by List, Monaco 'Achiral Counteranion Effects in an Asymmetric Iodolactonization' Synfacts, 2012, 8, 673.
53. Preparation of a Chiral Bis(Amidine) Ligand: Pyrrolidine Bis(Amidine)
Davis, T. ; Dobish, M. ; Schwieter, K. E. ; Chun, A. C. ; Johnston, J. N.
Org. Synth. 2012, 89, 380-393.
52. Umpolung Amide Synthesis: Discovery and Characterization of Anaerobic and Aerobic Pathways to Amide, Introducing an Opportunity for Straightforward Site-Selective 18O–Labeled Peptide Synthesis
Shackleford, J. ; Shen, B. ; Johnston, J. N.
Proc. Natl. Acad. Sci. 2012, 109, 44-46.
51. Total Synthesis of the Chlorine-Containing Hapalindoles K, A, G
Chandra, A. ; Johnston, J. N.
Angew. Chem. Int. Ed. 2011, 50, 7641-7644.
50. Enantioselective Organocatalysis in the Synthesis of Stilbene cis-Diamines: A Concise Preparation of (–)-Nutlin-3, a Potent p53/MDM2 Inhibitor
Davis, T. ; Johnston, J. N.
Chem. Sci. 2011, 2, 1076-1079.
Highlighted by Simon Hadlington in Chemistry World 2011 March 25.
Highlighted by Borman, S. 'Chiral Route To Key Anticancer Agent' Chemical & Engineering News, April 11, 2011, 89, 41.
Highlighted by 'Synthetic Protocol Published for Promising Anticancer Compound' Future Medicinal Chemistry 2011, 3, 779.
49. Origins of Selectivity in Brønsted Acid Promoted Diazoalkane-Azomethine Reactions (The aza-Darzens Aziridine Synthesis)
Troyer, T. ; Muchalski, H. ; Hong, K. ; Johnston, J. N.
Org. Lett. 2011, 13, 1790-1792.
48. Stereoselective Synthesis of Complex Polycyclic Aziridines: Use of the Brønsted Acid-Promoted aza-Darzens Reaction to Prepare an Orthogonally Protected Mitomycin C Intermediate with Maximal Convergency
Srinivasan, J. ; Mathew, P. ; Williams, A. L. ; Huffman, J. C. ; Johnston, J. N.
Chem. Commun. 2011, 47, 3975-3977.
47. Geometric Restraint Drives On- and Off-Pathway Catalysis by the Escherichia Coli Menaquinol:Fumarate Reductase
Tomasiak, T.M. ; Archuleta, T.L. ; Andrell, J. ; Luna-Chavez, C. ; Davis, T. ; Sarwar, M. ; Ham, A.J. ; McDonald, H. ; Yankovskaya, V. ; Stern, H.A. ; Johnston, J. N. ; Maklashina, E. ; Cecchini, G. ; Iverson, T.M.
J. Biol. Chem. 2011, 286, 3047-3056.
46. Brønsted Acid-Promoted Azide-Olefin Cycloadditions for the Preparation of Contiguous Aminopolyols Derived from an anti-1,3-Diol Scaffold: The Importance of Disiloxane Ring Size to Diastereoselection
Muchalski, H. ; Hong, K. ; Johnston, J. N.
Beilstein J. Org. Chem. 2010, 6, 1206-1210.
45. Transformations of Alkenes: Aziridination
Muchalski, H. ; Johnston, J. N.
Science of Synthesis 2011, 1, 155.
44. A Chiral N-Phosphinyl Phosphoramide: Another Offspring for the Sage Phosphoric Acid Progenitor
Johnston, J. N.
Angew. Chem. Int. Ed. 2011, 50, 2890-2891.
43. Chiral Brønsted Base-Promoted Nitroalkane Alkylation: Enantioselective Synthesis of Chiral Nonracemic sec-Alkyl-3-Substituted Indoles
Dobish, M. ; Johnston, J. N.
Org. Lett. 2010, 12, 5744-5747.
42. Preparation of Isopropyl 2-Diazoacetyl(phenyl)carbamate
Muchalski, H. ; Doody, A. ; Troyer, T. ; Johnston, J. N.
Org. Synth. 2011, 88, 212-223.
41. Umpolung Reactivity in Amide and Peptide Synthesis
Shen, B. ; Makley, D. ; Johnston, J. N.
Nature 2010, 464, 1027-1032.
Highlighted by Jaenicke, L. in Chemie in unserer Zeit 2010, 44, 319
Highlighted in Organic Process Research & Development 2010, 14, 1052-1060 (doi: 10.1021/op100229b)
Highlighted in Chemical & Engineering News, June 28, 2010 Borman, S. 'New Route to Amide Formation'
Highlighted by Scheidt, K. 'Organic chemistry: Amide bonds made in reverse' Nature 2010, 465 , 1020 (doi:10.1038/4651020a)
Highlighted in Pharmaceutical Technology August 2010, Van Arnum, P. 'Exploring Chiral Chemistry'
Highlighted in Pharmaceutical Technology May 2011, Van Arnum, P. 'A Marriage of Small Molecules and Biologics'
40. Bifunctional Asymmetric Catalysis: Amplification of Brønsted Basicity Can Orthogonally Increase the Reactivity of a Chiral Brønsted Acid
Davis, T. ; Wilt, J. C. ; Johnston, J. N.
J. Am. Chem. Soc. 2010, 132, 2880-2882.
39. To Protonate or Alkylate: Stereoselective Brønsted Acid Catalysis of Carbon-Carbon Bond Formation Using Diazoalkanes, Leading to Highly Functionalized Chiral Small Molecules
Johnston, J. N. ; Muchalski, H. ; Troyer, T.
Angew. Chem. Int. Ed. 2010, 49, 2290-2298.
38. A Brønsted Acid Catalyzed syn-Selective Glycolate Mannich Reaction
Troyer, T. ; Muchalski, H. ; Johnston, J. N.
Chem. Commun. 2009, 41, 6195-6197.
37. Use of Comparative Triazolinium Triflate Fragmentation Rates as a Tool to Assay Relative Competency of Brønsted Bases in N→N Proton Transfer
Donahue, M. ; Hong, K. ; Johnston, J. N.
Bioorg. Med. Chem. Lett. 2009, 19, 4971-4973.
36. Total Synthesis of the Lycopodium Alkaloid (+)-Serratezomine A
Chandra, A. ; Pigza, J. A. ; Han, J. ; Mutnick, D. ; Johnston, J. N.
J. Am. Chem. Soc. 2009, 131, 3470-3471.
Highlighted in Kocienski, P.; Schmidt, A. W. SynFacts 2009, 9, 946
35. A Formal Enantioselective Acetate Mannich Reaction: The Nitro Functional Group as a Traceless Agent for Activation and Enantiocontrol in the Synthesis of α-Amino Acids
Shen, B. ; Johnston, J. N.
Org. Lett. 2008, 10, 4397-4400.
34. A Diastereo- and Enantioselective Synthesis of α-Substituted anti-α,α-Diaminophosphonic Acid Derivatives
Wilt, J. C. ; Pink, M. ; Johnston, J. N.
Chem. Commun. 2008, 35, 4177-4179.
selected as ‘Hot Article’ by Chemical Communications reviewers and editor
33. A Preparation of Enantiomerically Enriched Axially Chiral β-Diketimines: Synthesis of (-)- and (+)-IAN Amine
Cortright, S. B. ; Counceller, C. ; Wilt, J. C. ; Perkins, B. ; Johnston, J. N.
Org. Lett. 2008, 10, 2445-2447.
32. A Diastereo- and Enantioselective Synthesis of α-Substituted syn-α,β-Diamino Acids
Singh, A. ; Johnston, J. N.
J. Am. Chem. Soc. 2008, 130, 5866-5867.
Highlighted in List, B.; Muller, S. SynFacts 2008, 7, 757
31. Free Radical-Mediated Aryl Amination: Convergent Two- and Three-Component Couplings to Chiral 2,3-Disubstituted Indolines
Viswanathan, R. ; Smith, C. R. ; Prabhakaran, E. ; Johnston, J. N.
J. Org. Chem. 2008, 73, 3040-3046.
30. On the Nature of Rate Acceleration in the Synthesis and Fragmentation of Triazolines by Brønsted Acid: Secondary Catalysis by Water (Hydronium Triflate)
Hong, K. ; Donahue, M. ; Johnston, J. N.
J. Am. Chem. Soc. 2008, 130, 2323-2328.
29. Synthesis of the ABC- and D-Ring Systems of the Indole Alkaloid Ambiguine G
Chandra, A. ; Viswanathan, R. ; Johnston, J. N.
Org. Lett. 2007, 9, 5027-5029.
28. Chiral Proton Catalysis: Enantioselective Brønsted Acid Catalyzed Additions of Nitroacetic Acid Derivatives as Glycine Equivalents
Singh, A. ; Yoder, R. A. ; Shen, B. ; Johnston, J. N.
J. Am. Chem. Soc. 2007, 129, 3466-3467.
27. Synthesis of an Advanced Intermediate En Route to Mitomycin C
Williams, A. L. ; Srinivasan, J. ; Johnston, J. N.
Org. Lett. 2006, 8, 6047-6049.
26. Preparation of a protected phosphoramidon precursor via an H-phosphonate coupling strategy
Donahue, M. ; Johnston, J. N.
Bioorg. Med. Chem. Lett. 2006, 16, 5602-5604.
25. Chiral Proton Catalysis: pKa Determination for a BAM-HX Brønsted Acid
Hess, A. ; Yoder, R. A. ; Johnston, J. N.
SynLett 2006, 1, 147-149.
24. A Case Study in Biomimetic Total Synthesis: Polyolefin Carbocyclizations to Terpenes and Steroids
Yoder, R. A. ; Johnston, J. N.
Chem. Rev. 2005, 105, 4730-4756.
23. Brønsted Acid-Promoted Olefin Aziridination and Formal anti-Aminohydroxylation
Mahoney, J. M. ; Smith, C. R. ; Johnston, J. N.
J. Am. Chem. Soc. 2005, 127, 1354-1355.
22. Free Radical-Mediated Aryl Amination: A Practical Synthesis of (R)- and (S)-7-Azaindoline-α-Amino Acid
Srinivasan, J. ; Burks, H. E. ; Smith, C. R. ; Viswanathan, R. ; Johnston, J. N.
Synthesis 2005, 2, 330-333.
21. A Remarkably Facile Zirconium(IV) to Aluminum(III) β-Diketiminate Transmetalation That Also Results in a More Active Olefin Polymerization Precatalyst
Cortright, S. B. ; Coalter III, J. N. ; Pink, M. ; Johnston, J. N.
Organometallics 2004, 23, 5885-5888.
20. IAN-Amines: Chiral C2-Symmetric Zirconium(IV) Complexes from Readily Modified Axially Chiral C1-Symmetric β-Diketimines
Cortright, S. B. ; Huffman, J. C. ; Yoder, R. A. ; Coalter III, J. N. ; Johnston, J. N.
Organometallics 2004, 23, 2238-2250.
19. Chiral Proton Catalysis: A Catalytic Enantioselective Direct Aza-Henry Reaction
Nugent, B. M. ; Yoder, R. A. ; Johnston, J. N.
J. Am. Chem. Soc. 2004, 126, 3418-3419.
Highlighted in Science Concentrates, Chemical & Engineering News, March 22, 2004
18. The Brønsted Acid-Catalyzed Direct Aza-Darzens Synthesis of N-Alkyl cis-Aziridines
Williams, A. L. ; Johnston, J. N.
J. Am. Chem. Soc. 2004, 126, 1612-1613.
Cortright, S. B. ; Yoder, R. A. ; Johnston, J. N.
Heterocycles 2004, 62, 223-227.
16. Free Radical-Mediated Vinyl Amination: A Mild, General Pyrrolidinyl Enamine Synthesis
Nugent, B. M. ; Williams, A. L. ; Prabhakaran, E. ; Johnston, J. N.
Tetrahedron 2003, 59, 8877-8888.
15. The First Azacyclopentenyl Carbinyl Radical Isomerizations (ACCRI): Independent Use of Steric and Electronic (Polarization) Effects as Gating Elements
Viswanathan, R. ; Mutnick, D. ; Johnston, J. N.
J. Am. Chem. Soc. 2003, 125, 7266-7271.
Highlighted in Science & Technology Concentrates, Chemical & Engineering News, June 9, 2003
14. Free Radical-Mediated Aryl Amination and its Use in a Convergent [3+2] Strategy for Enantioselective Indoline α-Amino Acid Synthesis
Viswanathan, R. ; Plotkin, M. A. ; Prabhakaran, E. ; Johnston, J. N.
J. Am. Chem. Soc. 2003, 125, 163-168.
13. Free Radical-Mediated Vinyl Amination: Access to N,N-Dialkyl Enamines and their β-Stannyl and β-Thio Derivatives
Prabhakaran, E. ; Williams, A. L. ; Nugent, B. M. ; Nailor, K. E. ; Johnston, J. N.
Org. Lett. 2002, 4, 4197-4200.
12. IAN-Amines: Direct Entry to a Chiral C2-Symmetric Zirconium(IV) β-Diketimine Complex
Cortright, S. B. ; Johnston, J. N.
Angew. Chem. Int. Ed. 2002, 41, 345-348.
11. Use of the vicinal Element Effect for Regiochemical Control of Quinone Substitutions and its Implication for Convergent Mitomycin Construction
Williams, A. L. ; Johnston, J. N.
Org. Lett. 2001, 3, 3695-3697.
10. Nonconventional Carbon Additions to Azomethines. Aryl Amination /Indoline Synthesis by Direct Aryl Radical Addition to Azomethine Nitrogen
Johnston, J. N. ; Plotkin, M. A. ; Viswanathan, R. ; Prabhakaran, E.
Org. Lett. 2001, 3, 1009-1011.
<script src="https://cdn.commoninja.com/sdk/latest/commonninja.js" defer></script> <div class="commonninja_component pid-f898b3f7-df4e-4a65-8f07-e01d225f94df"></div>